


Asymmetrex®’s Kinetic Stem Cell (KSC) Counting Technology is Featured in the Parent’s Guide to Cord Blood Foundation® June Newsletter
The Parent’s Guide to Cord Blood Foundation®’s monthly newsletter reports on research and development of medical treatments using blood and tissues from human umbilical cords in language that is accessible to parents of children receiving or considering such treatments. The June 14 issue of the newsletter features a graphical presentation of stem cell biotechnology company

Asymmetrex Describes How Advancing to Stem Cell-Specific Dosing May Begin with Veterinary Stem Cell Treatments
In a new book on Stem Cell Veterinary Science, stem cell biotechnology company Asymmetrex continues its effort to educate the field of human stem cell medicine on the need for stem cell-specific dosing to improve approved stem cell treatments and FDA-authorized stem cell clinical trials. A chapter contributed to the book by Asymmetrex President and

Asymmetrex Awarded Grant from ARMI BioFabUSA to Develop Tissue Stem Cell Counting Technology for Cell Biomanufacturing
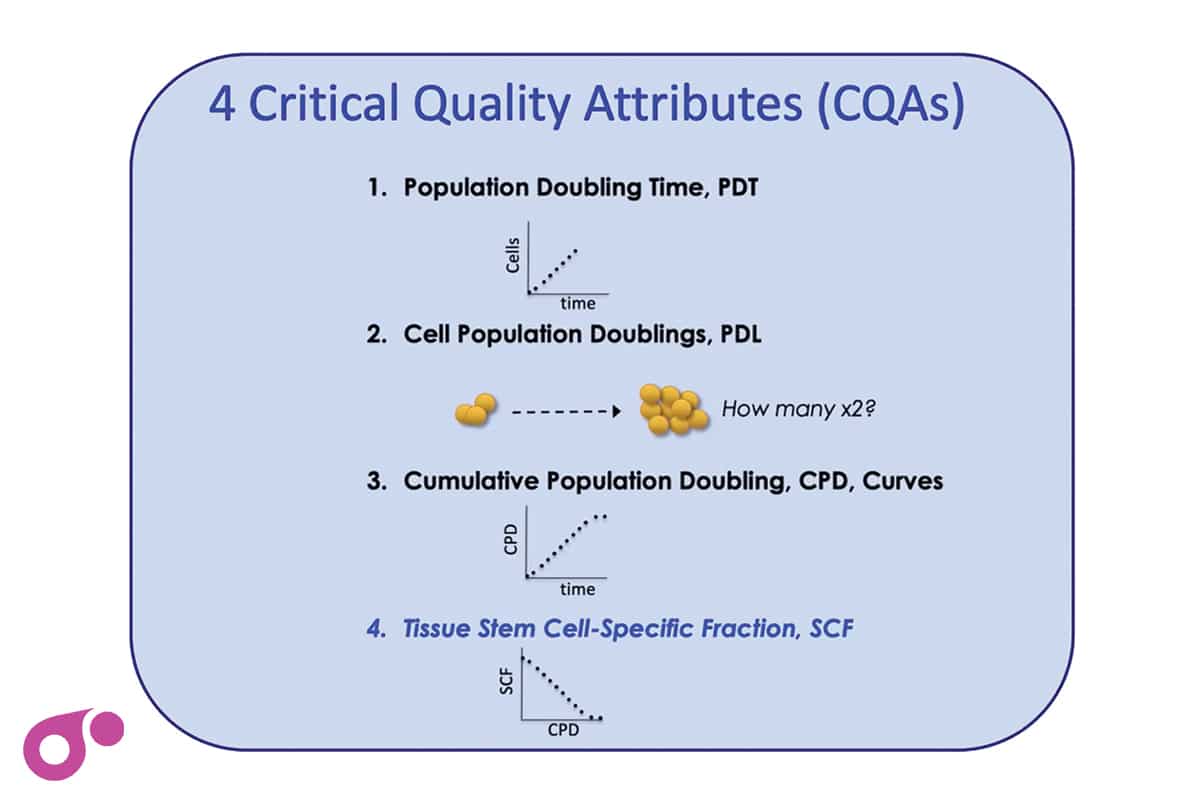
Stem cell biotechnology company Asymmetrex has been awarded a Technical Project grant from Advanced Regenerative Manufacturing Institute (ARMI) BioFabUSA. The new grant supports studies by Asymmetrex to evaluate its kinetic stem cell (KSC) counting technology as a method for determining a long-needed critical quality attribute for the biomanufacturing of potent therapeutic tissue stem cell-based products.
Asymmetrex Interviewed by SuperbCrew About New Web Portal With Tools for Therapeutic Stem Cell Applications
Last week on June 21 with the start of its participation in the virtual 2021 Annual Meeting of the International Society for Stem Cell Research, stem cell biotechnology company Asymmetrex launched a new web portal that provides scientists free online access to calculators for important parameters used in human tissue cell research. In a June 30 post, business and technology news platform SuperbCrew interviewed Asymmetrex’s

Asymmetrex Introduces Therapeutic Stem Cell Dosage Technologies to Biotherapeutics Bioprocessing Companies
Today, May 20, in the virtual 5th Annual MarketsandMarkets Bioprocessing of Biotherapeutics Conference, the President and CEO of stem cell biotechnology company Asymmetrex, James L. Sherley, M.D., Ph.D., introduced company executives and scientists to new technologies for determining the dosage of therapeutic stem cell products and treatments. A Gold Sponsor for the conference, Asymmetrex’s kinetic

Asymmetrex Presents the Value of Tissue Stem Cell Counting For Supplying Stem Cell Clinical Trials and Drug Development Clinical Trials
On September 30, stem cell biotechnology company Asymmetrex presented at the 2020 Outsourcing in Clinical Trials USA Virtual Conference. In addition to discussing the value of tissue stem cell counting for better practices in stem cell and gene therapy clinical trials, Asymmetrex director James Sherley also highlighted value that stem cell counting technologies can impart


